“Human Metapneumovirus: An Updated Review of Pathogenesis, Clinical Manifestations, and Therapeutic Approaches”
Tamara Ahmed Abd Alkareem.
dr.tamara1990@gmail.com
Mohammed Mahdi Sami.
mohamedmahdi81@kus.edu.iq
mataz J. Jamai.
mutaz.alwesy@kus.edu.iq
Remote Sensing Department, College of Remote Sensing and Geophysics, Al-Karkh University of Science, Baghdad, Iraq
Abstract:
Inflammation of the airways and trachea may be caused by the human metapneumovirus (hMPV). People with compromised immune systems, young kids, and elderly individuals are particularly at risk. Excessive fever, stuffy nose, difficulty breathing, and tiredness are the first signs of hMPV comparable to those of SARS-CoV-2. Globally dispersed research teams collaborate to further our understanding of hMPV’s clinical manifestations, preventative strategies, options for therapy, and diagnostic patterns. According to experts, the virus tends to spread over the winter. This study aims to thoroughly understand hMP, its clinical symptoms, preventative strategies, and experimental therapies for the virus. Unfortunately, hMPV vaccines do not currently exist.
Keywords: hMPV: human metapneumovirus, SARS-CoV-2: Severe acute respiratory syndrome coronavirus
فيروس التهاب الرئة البشري: مراجعة مُحدثة لعلم الأمراض، والمظاهر السريرية، والأساليب العلاجية“
تماره احمد عبدالكريم ، محمد مهدي سامي، معتز جليل جمعة
قسم التحسس النائي، كلية التحسس النائي والجيوفيزياء، جامعة الكرخ للعلوم
بغداد، العراق
الخلاصة:
قد يُسبب الفيروس الرئوي البشري (hMPV) التهاب المجاري الهوائية والقصبات الهوائية. يُعدّ الأشخاص ذوو المناعة الضعيفة، والأطفال الصغار، وكبار السن، أكثر عرضةً للخطر. تُعد الحمى الشديدة، واحتقان الأنف، وصعوبة التنفس، والتعب من أولى علامات الإصابة بفيروس hMPV، وهي أعراض تُشبه أعراض فيروس كورونا المُستجد (SARS-CoV-2). تتعاون فرق بحثية مُنتشرة عالميًا لتعزيز فهمنا للمظاهر السريرية لفيروس hMPV، واستراتيجياته الوقائية، وخيارات العلاج، وأنماط التشخيص. ووفقًا للخبراء، يميل الفيروس إلى الانتشار خلال فصل الشتاء. تهدف هذه الدراسة إلى فهم فيروس hMPV بشكل شامل، وأعراضه السريرية، واستراتيجياته الوقائية، وعلاجاته التجريبية. للأسف، لا توجد حاليًا لقاحات مُضادة لفيروس hMPV.
الكلمات المفتاحية: فيروس التهاب الرئة البشري (hMPV)، فيروس كورونا المسبب لمتلازمة الجهاز التنفسي الحادة الوخيمة (SARS-CoV-2).
Introduction:
People of every age are susceptible to the newly emerging respiratory infection known as human metapneumovirus (hMPV), primarily affecting youngsters, the elderly, and those with compromised immune systems (1,2). Along with respiratory syncytial virus (RSV), hMPV was first identified in 2001 and is a member of the Pneumoviridae family of negative-sense and single-stranded RNA isolated from preserved nasopharyngeal tissues; Van den Hoogen made his discovery in the Netherlands (5). The infection may spread when direct contact with secretions, such as spit or droplets, is the primary mode of infection transmission in kids and the elderly (3). The virus has an incubation period of three to six days (6). Primary symptoms that appear 5–14 days after RNA release are the onset of indicators that most persons with HPV infection may face, including (4). Pneumonia, fever, wheezing, cough, otitis media, purulent cough, bronchitis, nasal congestion, sore throat, dyspnea, Asthma that begins in adults, wheezing, difficulty breathing, hoarseness, coughing, and pneumonia are some of the more severe symptoms that may develop as the disease advances. This virus may cause bacterial pneumonia and bronchiolitis, the two most prevalent symptoms. Hospitalised children requiring specialised care for recovery from severe respiratory infections caused by hMPV make up around 5-10% of the total.
Age, co-occurring diseases (like hypertension, CVD, and other chronic illnesses), and pregnancy are among the many variables that influence the course of therapy. If, when infected, they have signs that are not entirely evident yet are consistent with acute bronchitis. Infected individuals might be either male or female. The vulnerability of the sexes is not significantly different. The signs of hMPV infection are similar to those of human respiratory syncytial virus, and it may cause severe bronchiolitis and pneumonia in children. Although most people get hMPV for the first time in their early infancy, re-infections may happen at any time. Approximately 5-10% of children hospitalised with severe respiratory infection had hMPV as the etiological agent. Although most people get hMPV for the first time in their early infancy, re-infections may happen at any time. Although individuals and those with weak immune systems are more likely to get hMPV infections, the virus mostly affects youngsters. Infection with hMPV may produce a wide variety of symptoms, from a minor infection of the upper respiratory tract to potentially fatal bronchiolitis and pneumonia. While hMPV and COVID-19 are distantly associated, they both infect the respiratory system; therefore, they may affect individuals of any age(7). Cough, fever, stuffy nose, and difficulty breathing are some of the symptoms often linked to hMPV. Infected individuals with SARS-CoV-2 also exhibit these indicators. Coughing, sneezing, and other respiratory secretions, as well as intimate proximity to humans, are the most common routes of transmission for both viruses. Another way viruses may spread is by direct contact with infected items or surfaces plus subsequent contact with the respiratory tract, eyes, or mouth(8).
Table (1): The table shows a comparison between Human Metapneumovirus & SARS-CoV-2(7).
|
Item |
Human Metapneumovirus | SARS-CoV-2 |
| Viral family | Paramyxoviridae | Coronaviridae |
| Genetic makeup
|
Single-stranded, negative-sense RNA |
Single-stranded, positive-sense RNA |
|
Transfer method
|
Intercourse involving contact with diseased skin
|
Intercourse involving contact with diseased skin
|
| Symptoms
|
Respiratory droplets – direct contact | Respiratory droplets – direct contact – contaminated surfaces |
| Groups most vulnerable to infection
|
Kids, older individuals, and individuals who have weakened immune systems.
|
This includes the elderly, those with compromised immune systems, and individuals who suffer from diabetes or cardiac conditions. |
| Duration of incubation
|
3 – 6 days |
2 -14 days |
|
Prevention methods
|
Washing hands – avoiding infectious persons – airing tight rooms. |
Influenza prevention measures include immunisation, mask use, social isolation, and handwashing. |
| Possible complications
|
Bronchitis – pneumonia – aggravation of respiratory illnesses. |
Syndrome of severe pneumonia, thrombosis, and polyinflammatory reactions. |
| Cases that require going to the hospital
|
It needs admission to the hospital when a significant loss of breath develops.
|
When severe signs such as difficulty breathing, low blood oxygen levels, organ failure, or worsening of preexisting conditions manifest, the situation becomes more precarious. |
Clinical Manifestations of hMPV:
Illness caused by hMPV often goes away within 2-5 days, much like a cold. Cough, high temperature, stuffy nose, difficulty breathing, and lethargy emerge 3-6 days after conception. Among ill children, pneumonia affects only a tiny fraction (5–16%)(9). The symptoms of a herpes simplex virus illness (HSV) may progress into bronchitis and are comparable to those of other respiratory diseases caused by viruses. In most cases, HMPV will cause the following symptoms: dyspnea, fever, a stuffy nose, and coughing. Asthma and pneumonia may all improve in sporadic cases due to sickness. Humans are a natural reservoir for this disease(10). The most common transmission routes are close personal contact, respiratory secretions, and not washing hands before contacting potentially infectious surfaces, which may transfer the virus to the body’s most sensitive organs. While HMPV often causes mild symptoms in healthy individuals, it has the potential to be lethal for some populations. Although the disease has a cyclical pattern, it has spread to every continent. In the Northern Hemisphere, disease season often begins in late winter (January) and peaks in March, but in the Southern Hemisphere, infection season typically begins in June and continues into July(11).
Virus prevention:
To avoid spreading the virus, it is recommended that patients remain home until their symptoms improve, as is the case with any respiratory illness. In addition to these basic precautions, people should wash their hands frequently with soap and water. Don’t touch their eyes, nose, or mouth unless they have washed them. Keep your distance from those with illness. Cover their mouth and nose when they cough or sneeze. Don’t share their food or drink with others. Don’t kiss or hug anyone. Stay home if you’re sick for your recovery(12).
Potential medications for the treatment of the human pneumonia virus:
Preventing receptors that aid in virus binding is one way antiviral medications stop the virus from integrating into healthy cells. Through cutting off this pathway, viruses are unable to infect cells. The following are examples of potential medicines used for managing the human pneumonia virus(13):
- Ribavirin:
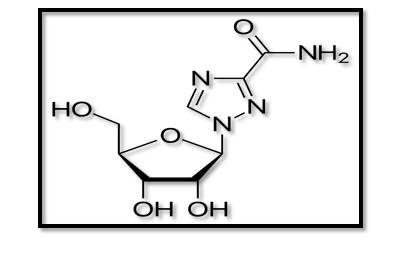
An antiviral medicine called ribavirin or tribavirin is utilised for treating viral illnesses such as hepatitis C virus (HCV), respiratory syncytial virus (RSV), as well as certain viral hemorrhagic fevers(14). On day 5, ribavirin considerably reduced global pulmonary inflammation and lung HMPV replication. Rapid and comprehensive recovery was achieved in an immunocompromised kid receiving chemotherapy for Burkitt’s lymphoma when intravenous immunoglobulin as well as oral ribavirin were administered together(15).

Figure (1): Structure of Ribavirin(16).
- Methiopropamine (MPA):
You may hear it called N-methylthiopropamine as well. This is one of the drugs that doctors recommend for pneumonia patients. The development of HMPV was significantly inhibited via MPA. The effectiveness of MPA in inhibiting SARS-COV-2 replication has been shown(17). Also, the new hallucinogenic chemical methylpropamine is structurally similar to methamphetamine. Mainly, it blocks the digestion of norepinephrine and dopamine, and secondly, it blocks the reuptake of serotonin. Methiopropamine is an energising and stimulating stimulant that improves attention as well as efficiency in people(18).
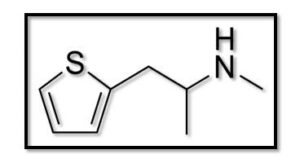
Figure (2): Structure of Methiopropamine(19).
- Psychedelic ( MPM ):
One less well-known psychedelic substance is MPM (2,5-dimethoxy-4-propoxyamphetamine), which is a modified amphetamine. Significant repression of HMPV replication MPM(20).
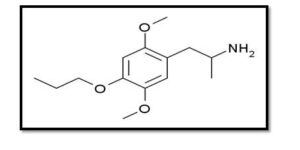
Figure (3): Structure of Psychedelic (21).
- Mycophenolate mofetil:
This is one of the potential inhibitors that may combat HPMV. The therapy for specific autoimmune illnesses and the prevention of tissue rejection after organ transplants are two of its most common uses. It has the properties of a tertiary amino acid, an ether, a carboxylic ester, a gamma-lactone, and a phenol. A 2-(morpholin-4-yl) ethanol and a mycophenolic acid are its functional relatives(22).
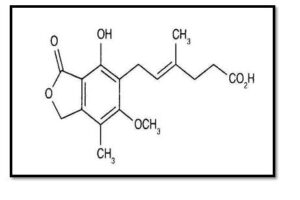
Figure (4): Structure of Mycophenolate mofetil (23).
- Monoclonal antibody:
Monoclonal antibodies, abbreviated as moAbs or mAbs, are man-made proteins that mimic the function of naturally occurring antibodies in the body. Displayed a substantial decrease in lung viral titres in mice following vaccination. It has also been demonstrated that human monoclonal antibodies can cure and avoid acute HMPV(24).
Conclusions:
Although it has only been identified recently, the human metapneumovirus (hMPV) seems to be just as deadly as the coronavirus. In the management of infection and the development of an effective vaccine against hMPV, one needs to know its pathophysiology and molecular restrictions for serious illness, since it is a significant respiratory pathogen. Unfortunately, hMPV vaccines do not currently exist. Antiviral drugs prevent integration into healthy cells by inhibiting receptors that help bind the virus to the cell. By preventing this connection, viruses cannot enter or infect the cell: Ribavirin, Methiopropamine (MPA), Psychedelic ( MPM ) and Monoclonal antibody.
Mice and hamsters have been used to evaluate all investigational therapies. What makes these medications intriguing, however, is that they include groups that are known to have antiviral activity; specifically, the amine (NH-) and alcohol (OH-) groups, which might play a role in the treatment of hMPV infections.
Acknowledgments:
This work would not have been possible without the financial as well as administrative backing of the Al-Karkh University of Science’s College of Remote Sensing & Geophysics and its Department of Remote Sensing.
References:
- Tulloch, R. (2023). The genomics of viral respiratory disease in Australia: Understanding the spread, evolution and pathogenesis of non-influenza respiratory viruses(Doctoral dissertation).
- Mousa, J. J., Williams, J. V., & Crowe Jr, J. E. (2023). Pneumoviruses: Respiratory Syncytial Virus and Human Metapneumovirus. In Viral Infections of Humans: Epidemiology and Control(pp. 1-53). New York, NY: Springer US.
- Peiris, J. M., & Madeley, C. R. (2020). Respiratory Viruses. Manson’s Tropical Diseases, 825.
- Dubey, A., Kumar, M., Tufail, A., & Dwivedi, V. D. (2025). Unlocking Antiviral Potentials of Traditional Plants: A Multi-Method Computational Study against Human Metapneumovirus (HMPV). bioRxiv, 2025-01.
- Panda, S., Mohakud, N. K., Pena, L., & Kumar, S. (2014). Human metapneumovirus: review of an important respiratory pathogen. International journal of infectious diseases, 25, 45-52.
- Divarathna, M. V., Rafeek, R. A., & Noordeen, F. (2020). A review on epidemiology and impact of human metapneumovirus infections in children using TIAB search strategy on PubMed and PubMed Central articles. Reviews in medical virology, 30(1), e2090.
- Heikkinen, T., Österback, R., Peltola, V., Jartti, T., & Vainionpää, R. (2008). Human metapneumovirus infections in children. Emerging infectious diseases, 14(1), 101.
- van den Hoogen, B. G., van Doornum, G. J., Fockens, J. C., Cornelissen, J. J., Beyer, W. E., Groot, R. D., … & Fouchier, R. A. (2003). Prevalence and clinical symptoms of human metapneumovirus infection in hospitalised patients. The Journal of Infectious Diseases, 188(10), 1571-1577.
- Yu, J., Xia, X., Chen, X., Wang, S., Zhang, X., & Cai, H. (2023). Clinical epidemiological features of hMPV infection in children with acute respiratory tract infection. Archives of Clinical Psychiatry, 50(6).
- Boivin, G., Abed, Y., Pelletier, G., Ruel, L., Moisan, D., Côté, S., … & Anderson, L. J. (2002). Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory tract infections in all age groups. The Journal of Infectious Diseases, 186(9), 1330-1334.
- Akingbola, A., Adegbesan, A., TundeAlao, S., Adewole, O., Ayikoru, C., Benson, A. E., … & Chuku, J. (2025). Human Metapneumovirus: an emerging respiratory pathogen and the urgent need for improved Diagnostics, surveillance, and vaccine development. Infectious Diseases, 57(3), 304-310.
- Van Den Bergh, A., Guillon, P., von Itzstein, M., Bailly, B., & Dirr, L. (2022). Drug repurposing for therapeutic discovery against human metapneumovirus infection. Antimicrobial Agents and Chemotherapy, 66(10), e01008-22.
- Wyde, P. R., Chetty, S. N., Jewell, A. M., Boivin, G., & Piedra, P. A. (2003). Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral research, 60(1), 51-59.
- Kitanovski, L., Kopriva, S., Pokorn, M., Dolnicar, M. B., Rajic, V., Stefanovic, M., & Jazbec, J. (2013). Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. Journal of pediatric haematology/oncology, 35(7), e311-e313.
- Kitanovski, L., Kopriva, S., Pokorn, M., Dolnicar, M. B., Rajic, V., Stefanovic, M., & Jazbec, J. (2013). Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. Journal of pediatric haematology/oncology, 35(7), e311-e313.
- Andrawis, M., Ellison, L. C., Riddle, S., Mahan, K., Collins, C. D., Brummond, P., & Carmichael, J. (2019). Recommended quality measures for health-system pharmacy: 2019 update from the Pharmacy Accountability Measures Work Group. American Journal of Health-System Pharmacy, 76(12), 874-887.
- Nguyen, P. T., Dang, D. K., Tran, H. Q., Shin, E. J., Jeong, J. H., Nah, S. Y., … & Kim, H. C. (2019). Methiopropamine, a methamphetamine analogue, produces neurotoxicity via dopamine receptors. Chemico-Biological Interactions, 305, 134-147.
- Tuv, S. S., Bergh, M. S. S., Andersen, J. M., Steinsland, S., Vindenes, V., Baumann, M. H., … & Bogen, I. L. (2021). Comparative neuropharmacology and pharmacokinetics of methamphetamine and its thiophene analogue methiopropamine in rodents. International Journal of Molecular Sciences, 22(21), 12002.12002.
- Brandt, S. D., Freeman, S., Fleet, I. A., & McGagh, P. (2013). “Analytical and Synthetic Aspects of Methiopropamine (MPA), a Thiophene-Based Stimulant.” Drug Testing and Analysis, 5(5), 291-295.
- Ullah, A., Khan, M., Zhang, Y., Shafiq, M., Ullah, M., Abbas, A., … & Diao, Y. (2025). Advancing Therapeutic Strategies with Polymeric Drug Conjugates for Nucleic Acid Delivery and Treatment. International Journal of Nanomedicine, 25-52.
- Halberstadt, A. L., & Geyer, M. A. (2013). Serotonergic hallucinogens as translational models relevant to schizophrenia. International Journal of Neuropsychopharmacology, 16(10), 2165-2180.
- Marchi, M., Farina, R., Rachedi, K., Laonigro, F., Žuljević, M. F., Pingani, L., … & Galeazzi, G. M. (2024). Psychedelics as an intervention for psychological, existential distress in terminally ill patients: A systematic review and network meta-analysis. Journal of Psychopharmacology, 02698811241303594.
- Dhossche, J., Simpson, E., & Hajar, T. (2017). Topical corticosteroid withdrawal in a pediatric patient. JAAD case reports, 3(5), 420-421.
- Hamelin, M. E., Gagnon, C., Prince, G. A., Kiener, P., Suzich, J., Ulbrandt, N., & Boivin, G. (2010). Prophylactic and therapeutic benefits of a monoclonal antibody against the fusion protein of human metapneumovirus in a mouse model. Antiviral research, 88(1), 31-37.